how to get rid of sulfur in water well

WQ-11
12/90
Sulfur Water Control (Rotten Egg Odor in Home Water Supplies)
Adel L. Pfeil
Department of Consumer Sciences and Retailing
Foul-smelling or unusual odors from your water should make you question its quality and safety. Some odors indicate the presence of contaminants which may pose a health risk. Other odors, such as those caused by hydrogen sul- fide, are more of a nuisance, only affecting the taste of the water.
Effects of Sulfur Water
Sulfur in your water supply is easily recognized by its offensive odor. Hydrogen sulfide gas causes the "rotten-egg" or sulfur water smell. Hydrogen sulfide in water causes no known health effects. However, high concentrations do change the taste of the water.
Hydrogen sulfide dissolved in water corrodes metals such as iron, steel, copper and brass. The corrosion of iron and steel from sulfur forms ferrous sulfide or "black water." Hydrogen sulfide in water can blacken silverware and discolor copper and brass utensils.
Sulfur water makes cleaning clothes very difficult. Using chlorine bleach in sulfur water reduces the cleaning power of detergent. Hydrogen sulfide in water also corrodes exposed metal parts in washing machines.
Iron and manganese, often present with hydrogen sulfide, turn the water black and greasy-feeling. If untreated, the water stains laundry, washing machines, sinks and kitchenware. When used in the laundry, chlorine bleach reacts with iron and manganese forming dark rusty or brownish stains on clothes.
Occurrence & Characteristics
Generally, hydrogen sulfide occurs in concentrations of less than 10 mg/l (milligrams per liter), but occasionally amounts of 50 to 75 mg/l are found. Hydrogen sulfide is more commonly found in ground water supplies than in sur- face water. Hydrogen sulfide gas quickly escapes from surface water.
Wells drilled in shale or sandstone, or near coal or oil fields often have hydrogen sulfide present. Much of the ground water in northwestern and northeastern Indiana has noticeable hydrogen sulfide levels. High levels of hydrogen sulfide also occur in smaller sections around the state (Figure 1).
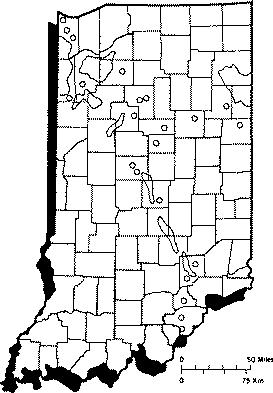
Figure 1. Areas of Indiana where hydrogen sulfide is found in ground water.
Hydrogen sulfide may also be produced when sulfate in well water converts to hydrogen sulfide. Certain non-disease-producing bacteria use the oxygen in the sulfate to form hydrogen sulfide.
Hydrogen sulfide causes the distinct, offensive odor of sewage. Occasionally, sewage pollution is the reason for the odor in drinking water. Sewage pollu- tion sulfide, and not a natural source, can occur in some surface water, in poorly constructed wells or in shallow wells close to sewer lines or septic tanks.
Under certain conditions, you may notice hydrogen sulfide when heating water. Heated water releases hydrogen sulfide gas quicker than cold water. A second situation occurs when sulfate in the water changes to hydrogen sulfide in the hot water heater. In this case, a magnesium rod has been installed in the water heater to reduce corrosion of the water heater. As the rod gives up small amounts of magnesium to the water, some hydrogen is released. The hydrogen can then combine with sulfur in the water and form hydrogen sulfide.
Hydrogen Sulfide Testing
The offensive odor of hydrogen sulfide generally makes testing unnecessary. Most people know when hydrogen sulfide is present and seek to correct the problem.
However, in a few cases, the odor may be from sewage pollution. Water with only hydrogen sulfide present does not cause disease. Sewage pollution, how- ever, contains disease-producing contaminants. When sewage pollution is a possible source of the sulfur, test your water for coliform bacteria.
Collect water samples for the hydrogen sulfide test at the well site. Hydro- gen sulfide gas easily escapes from water. Water testing labs and other pro- fessionals can provide further information on specific steps for testing.
Sulfide concentrations are reported as milligrams per liter (mg/l) or parts per million (ppm). These two terms are used interchangeably. Sulfide levels of 0.35 mg/l and less may go unnoticed. Amounts of 0.5 mg/l or more are usu- ally noticed, even in cold water. Some people become accustomed to the odor and tolerate hydrogen sulfide levels of 5 and 6 mg/l. Visitors might find such water very unpleasant, however.
Sulfur Removal Methods
Several methods of removing sulfur from water are available. The treatment method selected depends on many factors. These factors include the level of sulfur in the water, the amount of iron and manganese in the water, and if bacterial contamination must also be treated. Remember to consider the sim- plicity of the treatment method and the total cost including installation, maintenance and chemical costs.
Chlorine Bleach Removal Method
Chlorine bleach can effectively remove medium to high levels (over 6 mg/l) of hydrogen sulfide. The chlorine in the bleach chemically reacts with (oxi- dizes) the hydrogen sulfide eliminating the "rotten egg" odor. Chlorine bleach also reacts with iron or manganese, and disinfects water supplies.
An automatic chlorinator (chemical feed pump) adds chlorine to the water sys- tem (Figure 2). A filtering system then removes the sulfur, iron and man- ganese sediment formed. A settling tank sometimes replaces the filter system. A 500 to 1,000 gallon settling tank is generally sufficient.
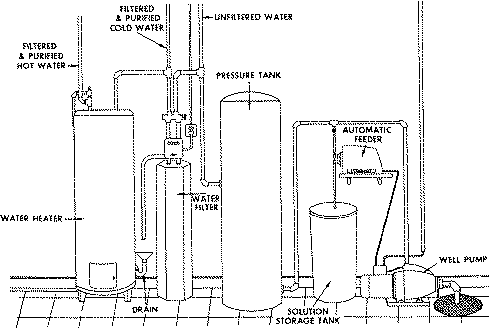
Figure 2. Example of an automatic chlorinator system.
Depending upon the amount of chlorine bleach added, a dechlorinating carbon filter may be used to obtain chlorine-free water for cooking and drinking. The same activated carbon filter can also remove the sulfur sediment. Mainte- nance and replacement of filter systems should be considered since sulfur, iron, manganese and other suspended materials in the water soon clog the filter.
Iron Removal Filter Method
An iron removal filter can remove low to moderate amounts (up to around 10 mg/l) of hydrogen sulfide in addition to removing iron and manganese. The filter oxidizes the hydrogen sulfide, converting it into insoluble sulfur which the filtering process then removes.
The filter needs to be regularly recharged with potassium permanganate. The complex recharging process makes the filter system very specialized. The ins- tallation and operation instructions of the manufacturer should be followed precisely. Because the precipitated sulfur can clog the filter, the filter needs regular replacement.
Aeration Removal Method
Aeration (adding air to the water), by itself, may not always reduce the hydrogen sulfide to non-detectable levels. However, the process sometimes reduces the hydrogen sulfide to acceptable amounts.
An aerator is installed between the well and a non-pressurized water storage tank (Figure 3). A diffuser, cascade or spray aeration system above the tank aerates the incoming water. The release of the water pressure and exposure to the air removes some of the sulfur compounds. Oxidation removes some of hydrogen sulfide gas. The process does produce the strong hydrogen sulfide odor near the aerator.
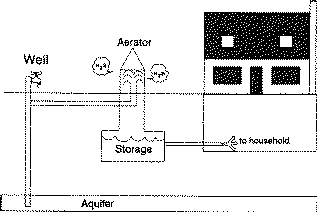
Figure 3. Aeration releases hydrogen sulfide and provides an acceptable water supply for the household.
A corrosion-resistant, screened vent under a water-tight roof should be installed. The storage tank and aeration system must be secure to prevent contamination of the water supply.
Operation of the aeration system requires good ventilation. The tank needs occasional cleaning as precipitated sulfur, iron sulfide, rust and algae col- lect. A valve controlled drain line to the ground surface makes flushing the storage tank, once or twice each year, easier.
Besides reducing the sulfur content, aeration also helps remove some of the iron, if present in the water. Oxidation of the iron occurs, and with enough settling time (holding the water in the tank for two to three days), satis- factory odor-free and iron-free water can be obtained. Activated carbon filters
Activated carbon filters absorb some hydrogen sulfide but have very limited capacity for major odor absorption. Carbon filters commonly are installed under the sink to treat drinking and cooking water. The filters must be removed and replaced often.
Other Considerations
Ordinary household water softeners do not remove sulfur odors from water. In fact, softeners easily become fouled or clogged, reducing their softening capacity. The exchange material may eventually need replacing.
Drilling a new well to find water with lower sulfur content may be a solution or be a waste of effort and money. Changing the depth of the well or moving a distance away from the original well, may not result in tapping different water-bearing layers (Figure 4). A different water-bearing layer may or may not be sulfur-free. Generally, the deeper the well, the higher the mineral and sulfur content.
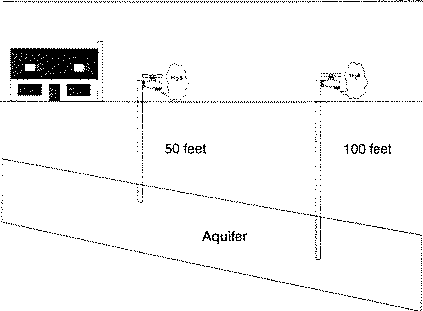
Figure 4. A new well may or may not have hydrogen sulfide in the water.
For Further Information
For further information on water quality contact your county Cooperative Extension office or local Health Department. The following bulletins in the WQ series may also be helpful:
- WQ 1 "Water Testing Laboratories"
- WQ 2 "What Is Ground Water?"
- WQ 3 "How to Take a Water Sample"
- WQ 4 "Why Test Your Water?"
- WQ 5 "Interpreting Water Test Results Part One: Inorganic Materials"
- WQ 6 "Buying Home Water Equipment"
- WQ 9 "Water Quality for Animals"
- WQ 10 "Wetlands and Water Quality"
- WQ 12 "Distillation for Home Water Treatment"
- WQ 13 "Home Water Treatment Using Activated Carbon"
- WQ 14 "Reverse Osmosis for Home Treatment of Drinking Water"
- WQ 16 "Bacterial Contamination of Household Water"
References
Clark, G. Douglas, ed., The Indiana Water Resource, Availability, Uses and Needs, Indiana Department of Natural Resources, Indianapolis, 1980, p. 86."Water Treatment Fundamentals", l983, Water Quality Association Educational Services.
This material is based upon work supported by the U.S. Department of Agriculture, Extension Service, under special project number 90-EWQI-1-9242.
Cooperative Extension work in Agriculture and Home Economics, state of Indiana, Purdue University, and U.S. Department of Agriculture cooperating; H. A. Wadsworth, Director, West Lafayette, IN. Issued in furtherance of the acts of May 8 and June 30, 1914. The Cooperative Extension Service of Purdue University is an affirmative action/equal opportunity institution.
how to get rid of sulfur in water well
Source: https://www.extension.purdue.edu/extmedia/wq/wq-11.html
Posted by: shafferwhow1970.blogspot.com

0 Response to "how to get rid of sulfur in water well"
Post a Comment